-
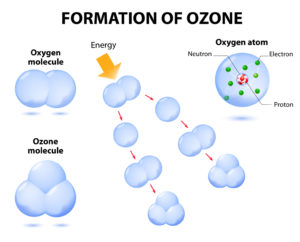
Ozone is a gas produced at the point of use in a device called an ozone generator utilizing oxygen-enriched feed gas and electricity
-
Oxygen molecules (O2) split with the addition of energy, resulting in two individual oxygen atoms (O1)
-
Oxygen atoms (O1) unite with other oxygen molecules (O2) to produce Ozone (O3)
-
(O1) + (O2) = (O3) as represented in the diagram
Ozone Properties
Ozone Properties Continued
-
The weak bond holding ozone’s third oxygen atom causes the molecule to be unstable and consequently a strong oxidizer, disinfectant and sanitizer
-
Ozone has a short half-life (seconds to minutes depending on temperature and pH if aqueous; minutes to hours in air), and reverts to oxygen
-
Ozone is not a chemical that can be stored; it must be generated and applied on-site
-
Ozone can be utilized as a gas (in a confined and controlled-access space) or it can be dissolved in water for targeted application
-
When ozone is utilized for surface sanitation, direct contact and CIP/SIP it is dissolved in water
-
Gaseous Ozone dissolved in water is referred to as Aqueous Ozone (which does not off-gas when applied appropriately)
-
When ozone comes into contact with a bacterium, the most active but most weakly bonded oxygen atom separates and oxidizes the cell membrane, ultimately causing cell bursting and destruction
-
The weak bond splits, leaving oxygen as a by-product
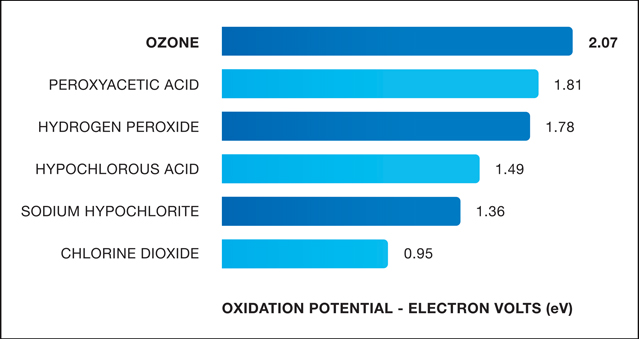
Oxidation Strength Comparison
Ozone Byproducts & Safety
-
The usual ozone by-products of organics (aldehydes, ketones, organic acids) are beneficial to the environment and (in the low levels formed during disinfection/sanitizing) are not toxic to people
-
Ozone leaves no residual on surfaces because of its short half-life
-
Ozone monitoring and system controls are always installed as part of an ozone generating and application unit
-
Ozone is a sustainable product with an over one-hundred year history of safe and practical commercial uses
Common Applications of Aqueous Ozone
-
Drinking water, bottled water, wastewater
-
Marine aquaria, aquaculture, laundries, pharmaceutical, ultrapure waterpreparation (electronics), water reuse
-
Pulp & paper bleaching, kaolin bleaching
-
Agriculture irrigation water, ground water remediation
-
Food processing and food service
Ozone as a Sanitizer
-
Ozone typically is applied as an aqueous product
-
It can be hard-plumbed into existing sanitation lines as a centralized system
-
It can be utilized with hand-held or fixed sprayers
-
It can also be used as a flood or cascade
-
Aqueous ozone is sprayed at low pressure (20 psi or less) in cold water (<70°F)
-
Low pressure use is designed to gently flood surfaces without causing pressurized over -spray that can inadvertently spread microorganisms to other areas of a facility and/or result in some off-gassing of ozone to plant air
Ozone Sanitizing Uses
-
Ozone-enriched cold water (aqueous ozone) can sanitize both food contact and non-food contact surfaces, as well as any other wettable area with sanitation needs
-
Floors, walls, conveyor belts, drains, tables, cutting boards, all flat or vertical surfaces
-
Flumes, tanks, carts, bins, basins
-
Ozone use will reduce levels of fat, oil & grease on surfaces, and it will break down microorganism and biofilm build -up on all surfaces
-
Its continuous use will sanitize floor drains with no adverse effect on wastewater treatment systems
-
Over time, ozone helps to rid drains and plumbing of biofilm and other microorganisms that can migrate back into the processi ng area (esp. Listeria monocytogenes)
-
Ozone is beneficial to sewage treatment systems because it adds dissolved oxygen to the wastewater to be treated
-
Regular use of aqueous ozone throughout the plant will help to eliminate greasy film on facility floors
-
Ozone can be safely used on wettable ceilings and walls to reduce and remove biofilms and molds (especially in areas of high sugar products)
-
Ozone sanitation sprays keep conveyor belts clean and free of build-up. “Build-up” may consist of food debris, sugar, fat, grease, etc., while harboring biofilm that may consist of any number of human pathogens, as well as fungi
-
Ozone is used to reduce bacterial buildup in RO modules
History Ozone Use in Food Facilities
-
Ozone-enriched cold water (aqueous ozone) can sanitize both food contact and non-food contact surfaces, as well as any other wettable area with sanitation needs
-
Floors, walls, conveyor belts, drains, tables, cutting boards, all flat or vertical surfaces
-
Flumes, tanks, carts, bins, basins
-
Ozone use will reduce levels of fat, oil & grease on surfaces, and it will break down microorganism and biofilm build -up on all surfaces
-
Its continuous use will sanitize floor drains with no adverse effect on wastewater treatment systems
-
Over time, ozone helps to rid drains and plumbing of biofilm and other microorganisms that can migrate back into the processi ng area (esp. Listeria monocytogenes)
-
Ozone is beneficial to sewage treatment systems because it adds dissolved oxygen to the wastewater to be treated
-
Regular use of aqueous ozone throughout the plant will help to eliminate greasy film on facility floors
-
Ozone can be safely used on wettable ceilings and walls to reduce and remove biofilms and molds (especially in areas of high sugar products)
-
Ozone sanitation sprays keep conveyor belts clean and free of build-up. “Build-up” may consist of food debris, sugar, fat, grease, etc., while harboring biofilm that may consist of any number of human pathogens, as well as fungi
-
Ozone is used to reduce bacterial buildup in RO modules
Ozone Sanitizing in Food Plants
Other facilities utilizing ozone for surface sanitation include:
-
Cheese processing plants; Eggs and Diary
-
Raw and RTE meat and poultry processing plants
-
Produce packers, produce processors
-
Seafood processors
-
Certified organic facilities
-
Ozone sanitizing is part of many food processors’ HACCP and HARPC programs
Ozone Sanitizing in Industrial Applications
Other facilities utilizing ozone for various sanitation processes include:
-
Pharmaceutical processing facilities
-
High purity water for biotech processors
-
High purity water for semiconductor producers
-
Cosmetic processors
-
Process Water Recycling
-
Anywhere wettable surfaces need to be sanitized
Ozone Safety & Control
Ozone SDS
Ozone (Gaseous)
-
OSHA PEL: 0.1 PPM8 hour
-
OSHA STEL: 0.3 PPM15 min
Ozone (Aqueous)
-
OSHA PEL: none established
-
OSHA STEL: none established
-
Eye Contact: may cause mild irritation; not expected
-
Ingestion Hazard: not ingested during application
-
Inhalation Hazard: not likely; exposure to aerosolized aqueous ozone could become irritating
-
Skin Contact: not hazardous
Aqueous ozone systems operated according to GMP, are safe for workers; these systems utilize monitor/control devices to continuously adjust operational parameters to ensure proper efficacy and safety
If catastrophic leaks happen, air monitor set point is exceeded; monitors instantly cut off electricity flow to ozone generators stopping production of ozone
Regulatory Summary -FDA
21 §CFR 129.80 (3/15/1977; amended 4/4/2012)
Bottled water plant sanitizing of contact surfaces and any other critical area
0.1 PPM ozone-enriched water solution for at least five minutes (Ct value of 0.5 mg-min)
21 CFR §173.368 (6/26/2001)
FDA Secondary Direct Food Additives Permitted in Food for Human Consumption
Ozone may be safely used in the treatment, storage, and processing of foods, including meat and poultry
Ozone is used as an antimicrobial agent in accordance with current industry standards of good manufacturing practice
21 §CFR 178.1010 (b) (1, 3, 9, 30, 38) (3/16/1977) “Category Three Certification”:
§178.1010 (b): “The solutions consist of one of the following, to which may be added components generally recognized as safe (GRAS) and components which are permitted by prior sanction or approval.”
-
(1) 200 PPM chlorine
-
(3) 25 PPM iodine (iodophore)
-
(9) 200 PPM quaternary ammonia compound
-
(30) 400-600 PPM peroxide
-
(38) 128-156 PPM peroxyacetic acid
Ozone is (GRAS) and listed under prior sanction (USEPA/FIFRA) Standard Dose 1-3 PPM Ozone
Regulatory Summary -USDA
November 27, 2001, the American Meat Institute filed a letter with USDA/FSIS requesting interpretation of the scope of the FDA rule allowing the use of ozone as an antimicrobial agent
USDA/FSIS determined that, “The use of ozone on raw and ready-to-eat meat and poultry products just prior to packaging is acceptable,”and that there are “no labeling issues in regard to treated product
USDA/FSIS Directive 7120.1 (12/17/02) (Revised 3/3/16)
“The attachment below identifies the substances that have been accepted since January 2000 by FSIS as safe and suitable for use in the production of meat and poultry products”
(Attachment 1) Antimicrobial-Ozone
-
All Meat and Poultry Products
-
In accordance with current industry standards of good manufacturing practice
-
Reference 21 CFR §173.368
USDA National Organic Program (NOP) Allowed
Ozone is listed in the NOP Final Rule (§205.605 (b) (20) pg. 437 -Nonagricultural (non organic) substances allowed as ingredients in or on processed products labeled as “organic”or “made with organic (specified ingredients or food group(s))”
-
(b) Synthetics allowed: (20) ozone
Regulatory –USDA -2
Prior to 1998, the USDA “White Book”listed authorized non-food surface contact compounds (including those for which sanitation claims were made) for meat or poultry
1998: NSF took responsibility for USDA “White Book”, updated listing, and
NSF encourages 3rd party testing to add candidates to the list and for approval by EPA OPP Disinfectant Tech Service Science Section (DIS/TSS) for no-rinse surface sanitation compliance
(Although recommended, this is not mandatory)
Regulatory Summary –EPA
-
EPA regulates ozone as a pesticide-producing device
-
Ozone generators must be registered by the EPA under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA)
-
Each Ozone Generator Manufacturer has a unique EPA registered establishment number as a pesticide-producing device
Regulatory –EPA -2
For no-rinse surface sanitation compliance the USEPA/FIFRA Office of Pesticide Programs (OPP) Disinfectant Technical Science Section (DIS/TSS) requires
-
Antimicrobial efficacy data determined by AOAC International methods
-
Toxicological
-
profiles
-
Environmental impact information
-
Specific label information and directions for use
Ozone Generators are recognized by the EPA as antimicrobial producing devices per EPA documentation published in 1976, with an EPA Establishment Number necessary for compliance.
A viable ozone system is compliant with items 1-4
Some Ozone Systems are listed in the “NSF White Book”
Regulatory Summary -OSHA
OSHA has two ozone standards to protect plant workers from exposure to harmful levels of ozone in facility air:
Permissible Exposure Level (PEL):
◦ 0.1 PPM ozone (by volume). Time-weighted average over an 8-hour work day, 5-days per week
Short Term Exposure Level (STEL):
◦ 0.3 PPM ozone (by volume) for no longer than 15-minutes, not to be exceeded more than four times per day.
These OSHA standards have been adopted worldwide wherever ozone is used commercially
Adherence to these allowable ozone exposures ensures that workers will never be exposed to toxic levels of gaseous ozone during working hours.
Antimicrobial Studies
Bacillus subtilis
-
“It is evident that ozone is superior to hydrogen peroxide in killing bacterial spores. Hydrogen peroxide at ~10,000-fold higher concentration was less effective than ozone against Bacillus spores. The comparatively low concentration needed to eliminate large populations of spores at ambient temperature in short time periods makes ozone best suited for industrial settings.”
- M.A. Khadre, A.E. Yousef, International Journal of Food Microbiology 71 (2001) 131–138
Antimicrobial Studies
B. cereus
-
0.12 mg/l @ 5 minutes (CT 0.6) @ 28°C = > 2 log
- M.A. Khadre, A.E. Yousef, International Journal of Food Microbiology 66 (2001) 1247
B. cereus
-
11.0 mg/l @ 1 minutes (CT 11.0) @ 22°C = > 6 log
- M.A. Khadre, A.E. Yousef, International Journal of Food Microbiology 71 (2001) 131
“Both studies provide statistical comparison only; therefore the ozone was not optimized, it is very likely more efficient and should be re-evaluated for optimum CT value and efficacy for Bacillus“
-Per Dr. Ahmed Yousef, February 2009
Electron Micrographs of E. coli before/after ozone treatment
Before ozone treatment | After ozone treatment

1. Ozone oxidizes cell membranes, causing osmotic bursting (instantaneously)
2. Ozone continues to oxidize enzymes and DNA
Air Liquide America Corp, Chicago Research Center, James T.C. YUAN, Ph.D., ca 2000
Equipment Surface Sanitation

Floor Surface Sanitation

Drain Surface Sanitation

Fish Conveyor w/ Ozone Spray

Fish Fillet and Skinner

Ozone Spray RTE Meat & Conveyor
Ozone Spray Sanitation on RTE Meat & Conveyor & Saw Blade
Chlorine vs. Ozone 11 Days Without Sanitation -Fruit

